
Actim® Partus
A rapid test to identify patients at risk of preterm or imminent birth.
Overview
A reliable way to assess the risk of preterm birth
Actim® Partus is a point-of-care test that can be used to reliably identify pregnancies with a real risk of preterm or imminent birth when the fetal membranes are intact.
Preterm birth (birth that occurs before the 37th week of pregnancy) is the leading cause of newborn morbidity and mortality. However, early detection of high-risk pregnancies can be a challenge: while symptoms related to preterm birth occur in as many as half of all pregnancies, only one in five women experiencing these symptoms are at real risk of immediate or preterm birth.
Actim Partus can be used from 22 weeks of gestation onwards. It provides reliable test results even in the presence of interfering substances such as semen, urine, infectious agents, bath or shower products.
Actim Partus in a nutshell

Can be used from week 22 onwards to rule out the risk of imminent or preterm birth.

The test result is available in just 5 minutes, with sampling completed in a matter of seconds.

Semen, urine, infectious agents, bath or shower products do not interfere with the test result.
Innovation behind Actim® Partus
Cervical phIGFBP-1: a specific marker to assess the risk of preterm birth
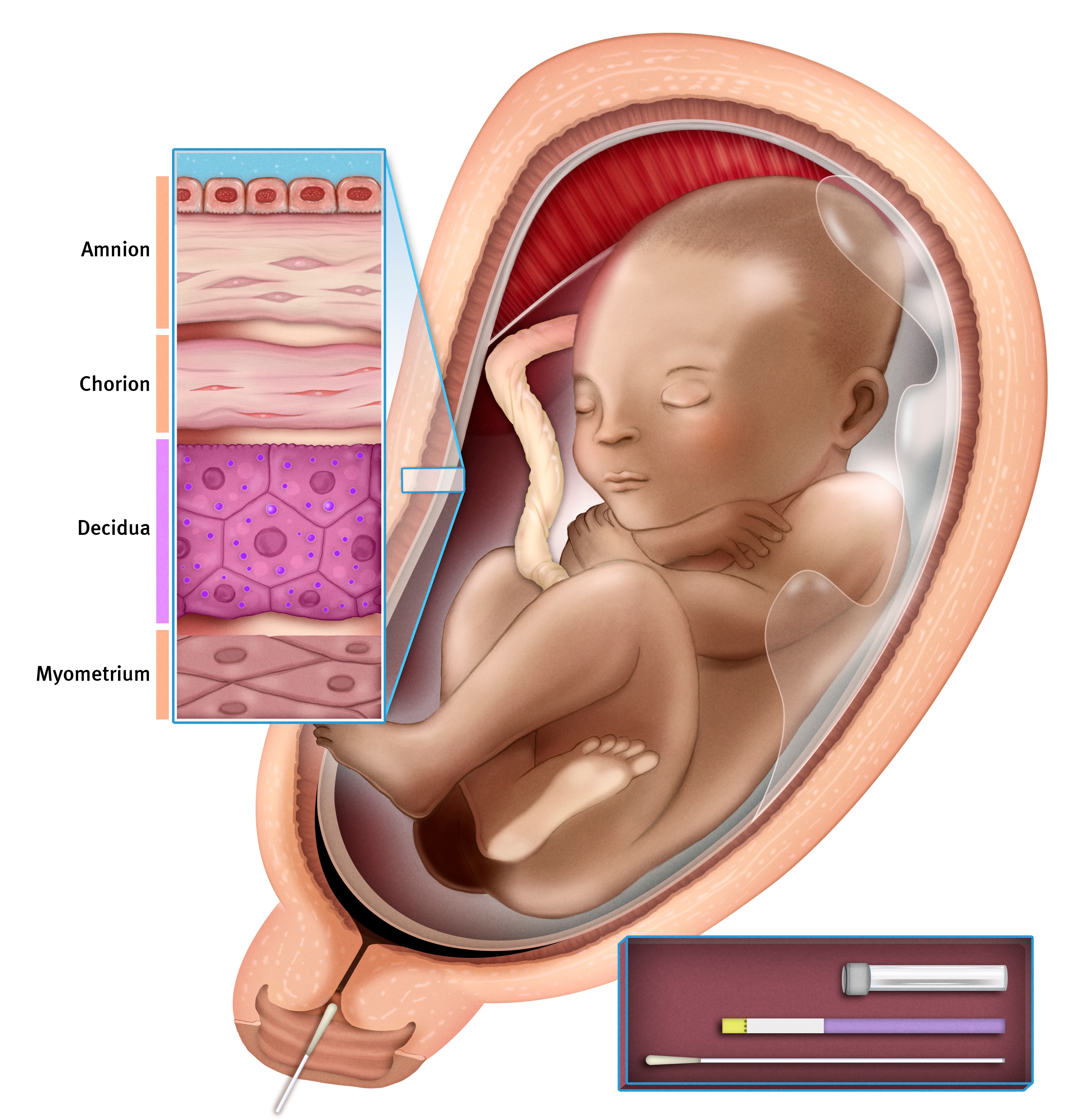
Actim Partus can be used to evaluate the risk of preterm birth with a cervical swab sample. The rapid test is based on highly specific monoclonal antibodies that bind to the phosphorylated form of insulin-like growth factor binding protein-1 (phIGFBP-1). phIGFBP-1 is produced in the decidua, and it leaks into the cervix when the decidua and chorion detach.
A positive test result indicates the presence of tissue damage, which may lead to preterm birth. A negative test result, however, means that there are no significant changes in the choriodecidual layer. The patient is therefore very unlikely to go into labour in the next 1 to 2 weeks and can be discharged, unless otherwise clinically indicated. Actim Partus is designed to give reliable answers in a matter of minutes to support clinical decision-making.
Because Actim Partus has a very high (98%) negative predictive value (NPV), a negative test result reliably rules out the risk of imminent or preterm birth.
About phIGFBP-1
- phlGFBP-1 is produced in decidua.
- It leaks into the cervix when decidua and chorion detach.
- phIGFBP-1 in a cervical swab is an indicator for tissue damage.
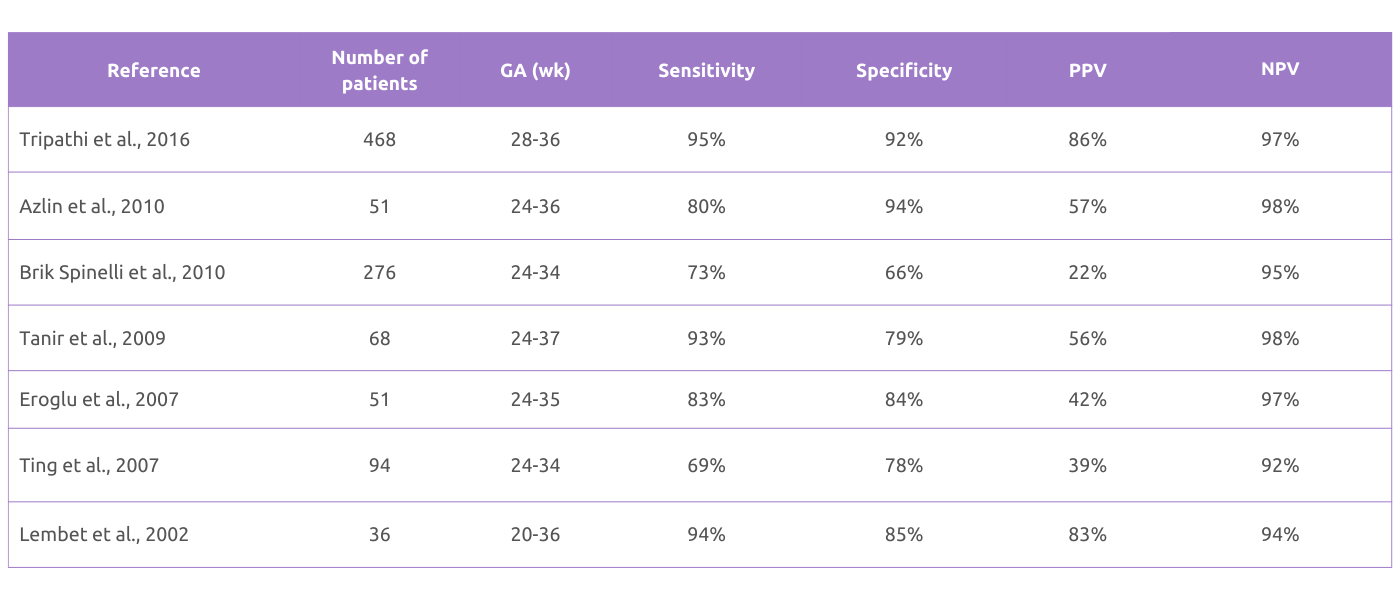
Table: Clinical evidence of Actim Partus as a predictor of birth within 7 days.

Preterm birth or Braxton Hicks contractions?
Identifying whether the patient is having Braxton Hicks contractions or is at real risk of preterm birth can be difficult. This is why we have developed Actim Partus, with test results available at the bedside in just 5 minutes.
Actim Partus is already in use across the globe, and it has been included in national treatment guidelines.
Actim Partus saves time and valuable resources

Early identification of high-risk pregnancies allows timely treatment.

Medical attention can be directed to patients who need it most with no delay.

Low-risk patients who do not need immediate care can be discharged to home.
How to use the test
Results available in 5 minutes at the bedside
The Actim Partus test kit contains all the necessary items, and it can conveniently be stored at room temperature (+2… +25 °C).
Learn how to use the test in the video below:
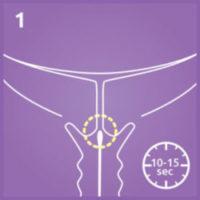
1. Collect a cervical secretion sample with a speculum*.
Hold the swab in the external o.s for 10-15 seconds.
*If Actim Partus is combined with Actim PROM, separate samples need to be taken for the two tests. The sample for Actim Partus test must be taken from the cervical o.s, while a a cervicovaginal sample from posterior fornix is used for Actim PROM test.
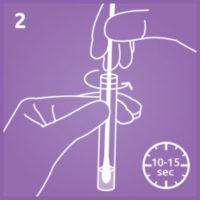
2. Extract the specimen.
Insert the swab into the extraction buffer. Swirl vigorously for 10-15 seconds and discard the swab.
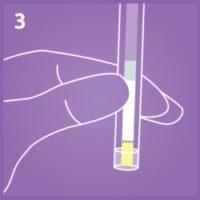
3. Activate the test.
Place the yellow dip area into the extracted specimen.
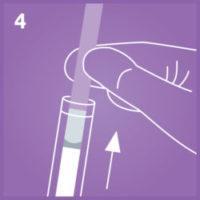
4. Remove the dipstick from the extracted specimen.
Remove the dipstick from the buffer once you see the liquid front enter the result area and place it on horizontal position.
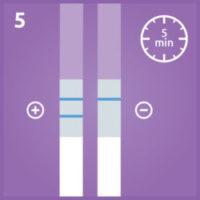
5. Interpret the test result:
- A positive result can be read as soon as it becomes visible.
Two blue lines = increased risk for preterm or imminent birth.
- A negative result should be confirmed at 5 minutes.
One blue line = preterm or imminent birth is highly unlikely to be happening within 7-14 days.

Product information
Actim Partus
The Actim Partus test kits are available in multiple package sizes. All test kits contain one Instructions for Use booklet and tests that are individually packed in pouches.
Each of the pouches include:
- 1 Dipstick
- 1 Extraction buffer tube
- 1 Sterile polyester swab

Actim Partus Controls
The Actim Partus Controls are intended to be used with Actim Partus for quality control purposes. The controls may also be used to demonstrate negative and positive test results.
The controls set includes negative, low positive and high positive controls as well as a reconstitution solution.
Product codes
- Actim Partus, 10 tests | product code 31931ETAC
- Actim Partus, 1 test | product code 31930ETAC
- Actim Partus Controls | product code 31900ETAC
Materials
Downloadable materials

- Brochures & leaflets
- Brochure
- Technical specifications leaflet
- Actim Partus and Actim PROM flowchart
- Instructions for use
- Actim Partus instructions for use
- Actim Partus Controls instructions for use
- Scientific materials
- List of publications mentioning Actim Partus
- Material safety data sheet
- If you wish to receive a Material safety data sheet, please contact regulatory@actimtest.com
Actim® Partus is not registered in the USA.
MDSAP 13485 and ISO 13485 certificates can be found here.